Adult Neurogenesis Is Altered by Circadian Phase Shifts and the Duper Mutation in Female Syrian Hamsters
Por um escritor misterioso
Last updated 19 março 2025

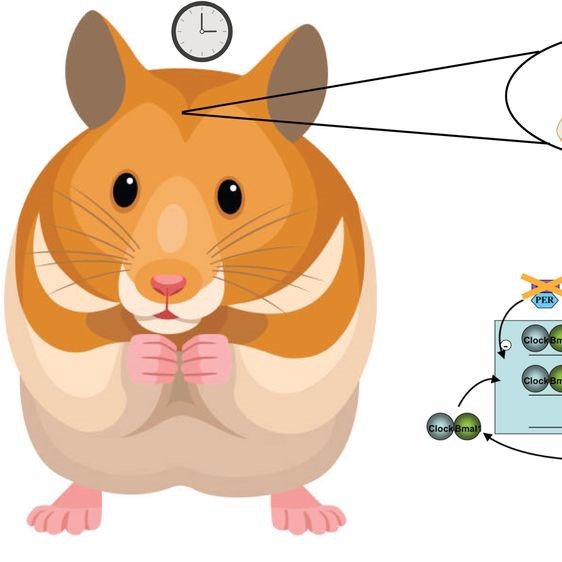
Cell birth and survival in the adult hippocampus are regulated by a circadian clock. Rotating shift work and jet lag disrupt circadian rhythms and aggravate disease. Internal misalignment, a state in which abnormal phase relationships prevail between and within organs, is proposed to account for adverse effects of circadian disruption. This hypothesis has been difficult to test because phase shifts of the entraining cycle inevitably lead to transient desynchrony. Thus, it remains possible that phase shifts, regardless of internal desynchrony, account for adverse effects of circadian disruption and alter neurogenesis and cell fate. To address this question, we examined cell birth and differentiation in the duper Syrian hamster ( Mesocricetus auratus ), a Cry1 -null mutant in which re-entrainment of locomotor rhythms is greatly accelerated. Adult females were subjected to alternating 8 h advances and delays at eight 16 d intervals. BrdU, a cell birth marker, was given midway through the experiment. Repeated phase shifts decreased the number of newborn non-neuronal cells in WT, but not in duper hamsters. The duper mutation increased the number of BrdU-IR cells that stained for NeuN, which marks neuronal differentiation. Immunocytochemical staining for proliferating cell nuclear antigen indicated no overall effect of genotype or repeated shifts on cell division rates after 131 days. Cell differentiation, assessed by doublecortin, was higher in duper hamsters but was not significantly altered by repeated phase shifts. Our results support the internal misalignment hypothesis and indicate that Cry1 regulates cell differentiation. Phase shifts may determine neuronal stem cell survival and time course of differentiation after cell birth. Figure created with BioRender.

Dysrhythmia in the suprachiasmatic nucleus inhibits memory

Graphs illustrating the temporal organization (a–c), phase

Reentrainment Impairs Spatial Working Memory until Both Activity

Experience dictates stem cell fate in the adult hippocampus

The duper mutation reveals previously unsuspected functions of

Photic Resetting and Entrainment in CLOCK-Deficient Mice - Robert

Phenotype of mPER2-expressing cells in the adult dentate gyrus

mPer2 mutation increased in vitro neural stem/progenitor cell
eNeuro Blog

Jet Lag Disrupts Brain Neurons

The circadian clock in adult neural stem cell maintenance

Actograms (left) and corresponding χ 2 periodograms (right) of

The duper mutation reveals previously unsuspected functions of
Recomendado para você
-
 Teddy Bear Hamster Animal Facts Mesocricetus auratus - A-Z Animals19 março 2025
Teddy Bear Hamster Animal Facts Mesocricetus auratus - A-Z Animals19 março 2025 -
 Viruses, Free Full-Text19 março 2025
Viruses, Free Full-Text19 março 2025 -
 Pika Life Cycle Clipart Set Download - Clipart 4 School19 março 2025
Pika Life Cycle Clipart Set Download - Clipart 4 School19 março 2025 -
 Rat Lifecycle — APC Management19 março 2025
Rat Lifecycle — APC Management19 março 2025 -
 Life Cycle of a Guinea Pig (Heinemann by Royston, Angela19 março 2025
Life Cycle of a Guinea Pig (Heinemann by Royston, Angela19 março 2025 -
 Hamsters - Exotic and Laboratory Animals - Merck Veterinary Manual19 março 2025
Hamsters - Exotic and Laboratory Animals - Merck Veterinary Manual19 março 2025 -
 Hamster Care: The Essential Guide to Ownership, Care, & Training For Your Pet: Pellham, Kate H: 9781511972406: : Books19 março 2025
Hamster Care: The Essential Guide to Ownership, Care, & Training For Your Pet: Pellham, Kate H: 9781511972406: : Books19 março 2025 -
 Life Cycle of Syrian Hamster, Life Cycle of Hamsters, Life Cycle of teddy bear Hamster,19 março 2025
Life Cycle of Syrian Hamster, Life Cycle of Hamsters, Life Cycle of teddy bear Hamster,19 março 2025 -
 A Hamster's Life Cycle (Everything You Need to Know) - Fur, Wings, & Scaly Things19 março 2025
A Hamster's Life Cycle (Everything You Need to Know) - Fur, Wings, & Scaly Things19 março 2025 -
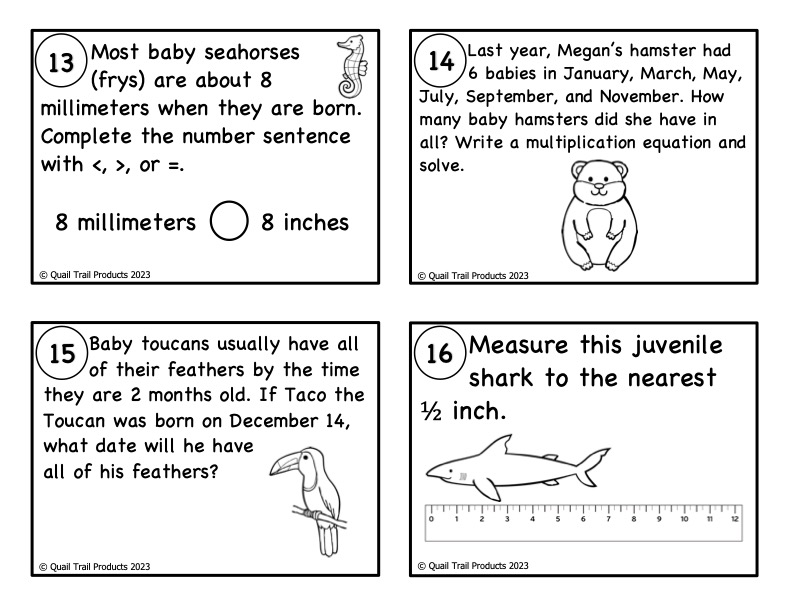 Life Cycle Math Task Cards - Classful19 março 2025
Life Cycle Math Task Cards - Classful19 março 2025
você pode gostar
-
/i.s3.glbimg.com/v1/AUTH_08fbf48bc0524877943fe86e43087e7a/internal_photos/bs/2021/a/i/1B0EluQauBtlKpvwBS6g/lancamentos-semana-super-mario-3d-world-bowsers-fury.jpg) Super Mario e Little Nightmares 2 são destaques nos lançamentos da19 março 2025
Super Mario e Little Nightmares 2 são destaques nos lançamentos da19 março 2025 -
 Dragon Ball GT: The Never-Seen Transformations of Cell and Frieza - Meristation19 março 2025
Dragon Ball GT: The Never-Seen Transformations of Cell and Frieza - Meristation19 março 2025 -
 Email Marketing: The Ultimate Guide (+ Expert Tips)19 março 2025
Email Marketing: The Ultimate Guide (+ Expert Tips)19 março 2025 -
Nup Gourmet - Bolo temático @Nupgourmet 🥰 Pra aqueles viciados em joguinhos, tema #ludochallenge #Bolo #bolosvgcuiabamt #confeitariacomamor #feitoamao #cuiaba #varzeagrande19 março 2025
-
 5 jogos de tabuleiro para você praticar japonês com seus amigos19 março 2025
5 jogos de tabuleiro para você praticar japonês com seus amigos19 março 2025 -
 Hanazawa Kana - voicing best girls since 2003 : r/anime19 março 2025
Hanazawa Kana - voicing best girls since 2003 : r/anime19 março 2025 -
 Undertale Flowey Pixel Art Minecraft Video Games, PNG, 1200x1200px, Undertale, Area, Art, Brand, Deviantart Download Free19 março 2025
Undertale Flowey Pixel Art Minecraft Video Games, PNG, 1200x1200px, Undertale, Area, Art, Brand, Deviantart Download Free19 março 2025 -
 Walkthrough:The Talk of Pirates, Sly Cooper Wiki19 março 2025
Walkthrough:The Talk of Pirates, Sly Cooper Wiki19 março 2025 -
 Gravel 20mm - Rowebb19 março 2025
Gravel 20mm - Rowebb19 março 2025 -
 Kawaii Galaxy Cat 3 Sticker – Mushimoo19 março 2025
Kawaii Galaxy Cat 3 Sticker – Mushimoo19 março 2025
