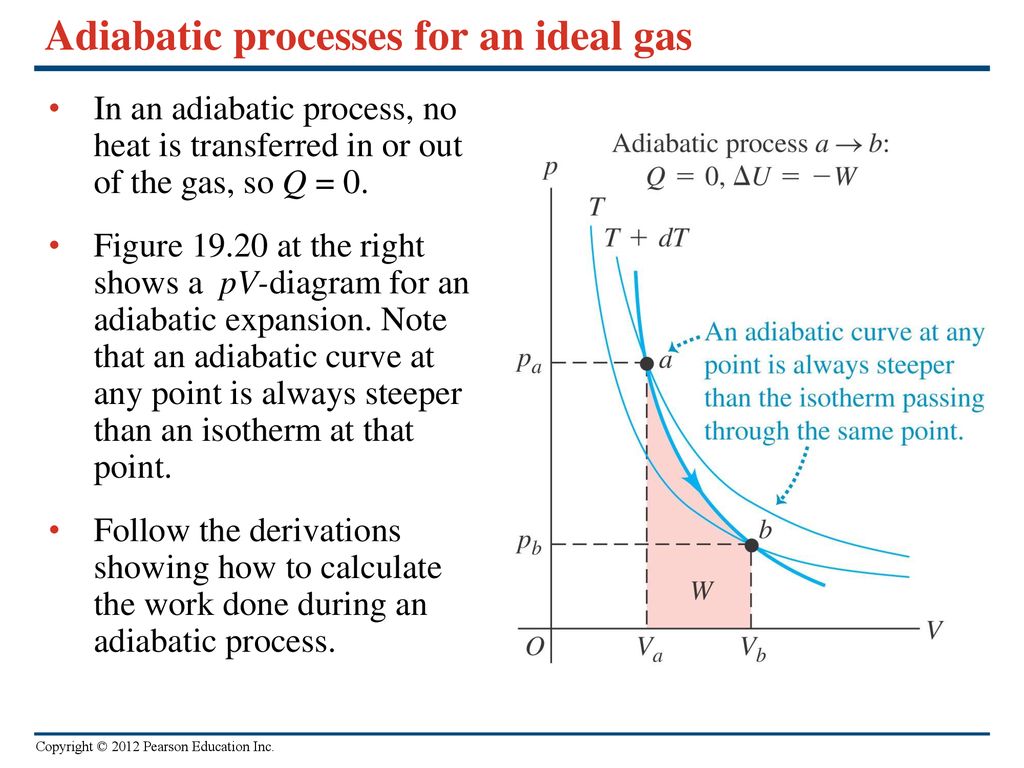
Why is Adiabatic Curve steeper than Isothermal Curve
Por um escritor misterioso
Last updated 12 março 2025

Compartilhe seus vídeos com amigos, familiares e todo o mundo
The First Law of Thermodynamics - ppt download
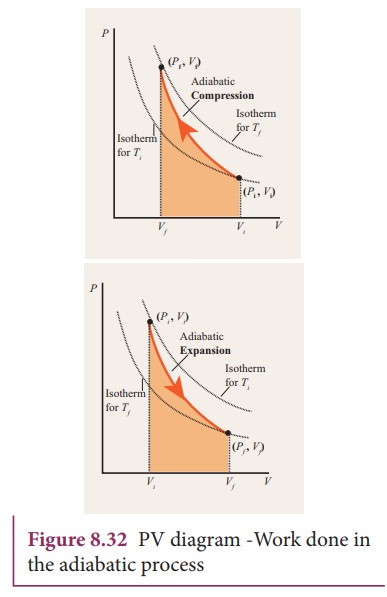
Adiabatic process - Thermodynamics

The incorrect figures representing isothermal and adiabatic expansion of an ideal gas from a particular initial state isare:BD
Why is an adiabatic curve more steeper than an isothermal curve? - Quora
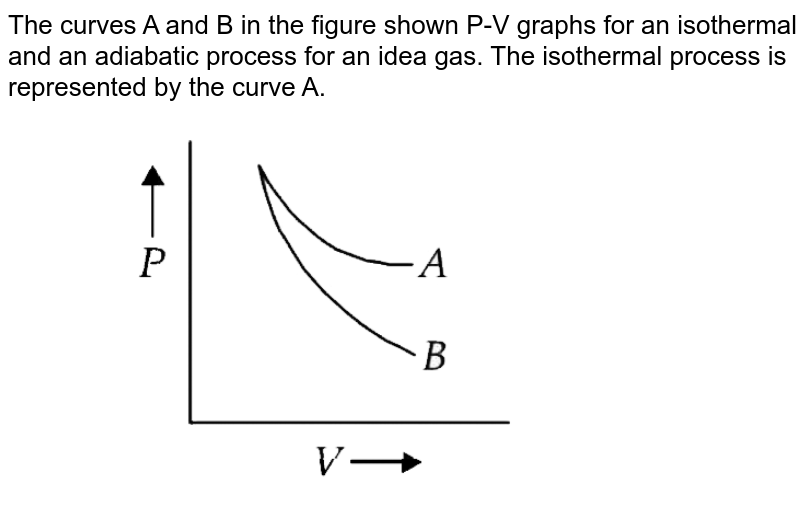
What is the ratio of slopes of P-V graphs of adiabatic and isother
Slope of which curve in PV graph is greater Adiabatic or isothermal? Which graph is more steeper Adiabatic or Isothermal? - ACE CHEMISTRY - Quora

thermodynamics - Why is the curve of an isothermal process above the that of a adiabatic process during compression? - Chemistry Stack Exchange

Adiabatic curve is steeper than the isothermal one explain.

IIT JEE - Thermodynamic Processe 2 (a) Offered by Unacademy
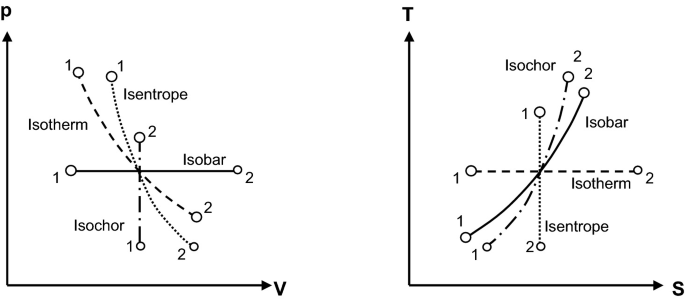
Thermodynamics

Show that an adiabatic curve is always steeper than an isothermal curve.
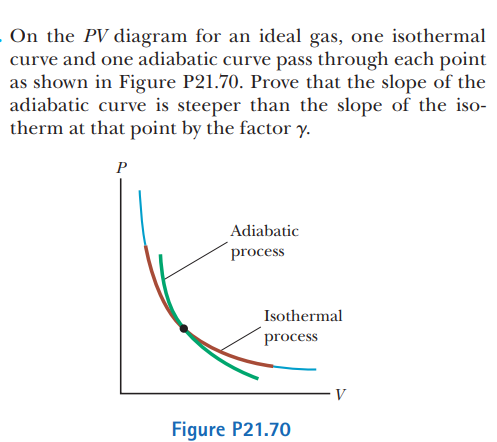
SOLVED: On the P V diagram for an ideal gas, one isothermal curve and one adiabatic curve pass through each point as shown in Figure P21.70. Prove that the slope of the

AP Physics B Ch. 12: Laws of Thermodynamics. Internal energy (U) Sum of the kinetic energy of all particles in a system. For an ideal gas: U = N K ave. - ppt download

In P-V graph of an ideal gas, which describe the adiabatic process

Adiabatic changes - Poisson equations
Recomendado para você
-
 a) A steeper, more negative (lower) gradient with a higher constant12 março 2025
a) A steeper, more negative (lower) gradient with a higher constant12 março 2025 -
 unity game engine - Math: Creating a curve with a steeper increase in value and longer ease in - Stack Overflow12 março 2025
unity game engine - Math: Creating a curve with a steeper increase in value and longer ease in - Stack Overflow12 março 2025 -
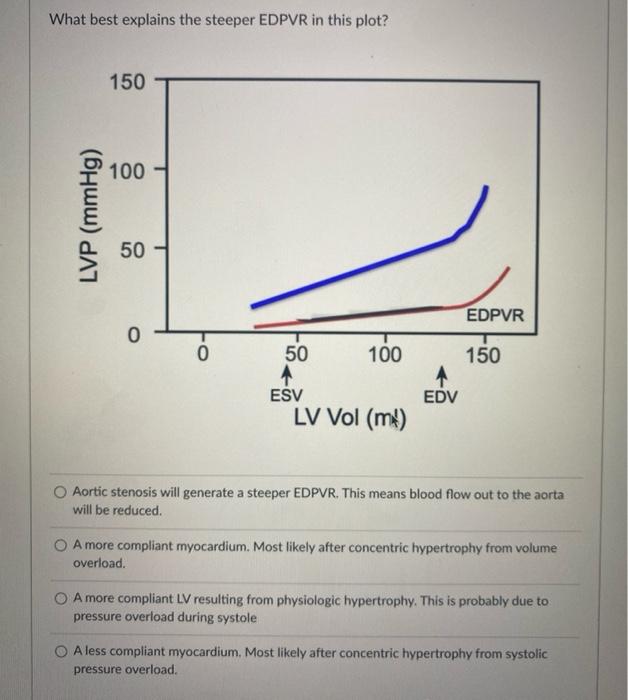
Solved What best explains the steeper EDPVR in this plot?12 março 2025
-
 Steeper Trend Line Trading System12 março 2025
Steeper Trend Line Trading System12 março 2025 -
Steeper Energy12 março 2025
-
 Steeper Trend Line With V-Power12 março 2025
Steeper Trend Line With V-Power12 março 2025 -
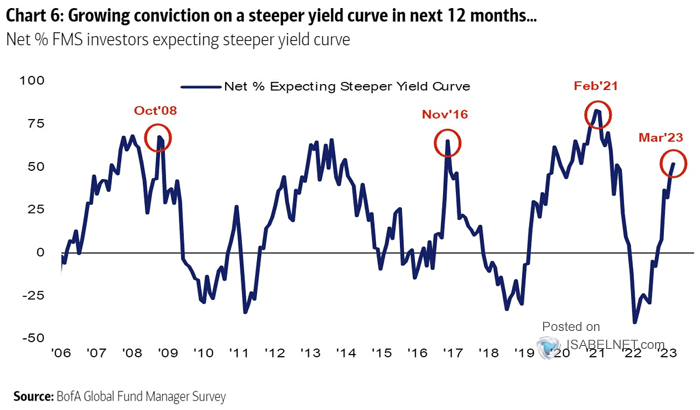 FMS Investors – Net % Expecting Steeper Yield Curve – ISABELNET12 março 2025
FMS Investors – Net % Expecting Steeper Yield Curve – ISABELNET12 março 2025 -
 Steeper Group - SteeperUSA12 março 2025
Steeper Group - SteeperUSA12 março 2025 -
 Oliver Steeper: Grieving parents condemn Boris Johnson's childcare plans - BBC News12 março 2025
Oliver Steeper: Grieving parents condemn Boris Johnson's childcare plans - BBC News12 março 2025 -
 Tuffy Steeper – Tumblewood Teas12 março 2025
Tuffy Steeper – Tumblewood Teas12 março 2025
você pode gostar
-
 Protetor Whelling Stunt Titan 160 Bráz Acessórios12 março 2025
Protetor Whelling Stunt Titan 160 Bráz Acessórios12 março 2025 -
 Las mejores ofertas en Botas Marrones de Mujer Louis Vuitton12 março 2025
Las mejores ofertas en Botas Marrones de Mujer Louis Vuitton12 março 2025 -
 Jogo · FNF VS Pou · Jogar Online Grátis12 março 2025
Jogo · FNF VS Pou · Jogar Online Grátis12 março 2025 -
 Spy Classroom: Data de estreia da 2ª temporada é divulgada12 março 2025
Spy Classroom: Data de estreia da 2ª temporada é divulgada12 março 2025 -
 Al-Ettifaq FC - Wikipedia12 março 2025
Al-Ettifaq FC - Wikipedia12 março 2025 -
 Conviction (Tom Clancy's Splinter Cell, #5): 9783833220531 - AbeBooks12 março 2025
Conviction (Tom Clancy's Splinter Cell, #5): 9783833220531 - AbeBooks12 março 2025 -
 Você já viu um cavalo sorrindo? - Momento Equestre12 março 2025
Você já viu um cavalo sorrindo? - Momento Equestre12 março 2025 -
 Max: All about HBO and Discovery's new streaming service - Home of internet privacy12 março 2025
Max: All about HBO and Discovery's new streaming service - Home of internet privacy12 março 2025 -
Sligo Sub Aqua Club12 março 2025
-
 Ijiranaide, Nagatoro-san 2nd Attack12 março 2025
Ijiranaide, Nagatoro-san 2nd Attack12 março 2025