FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 11 novembro 2024

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.
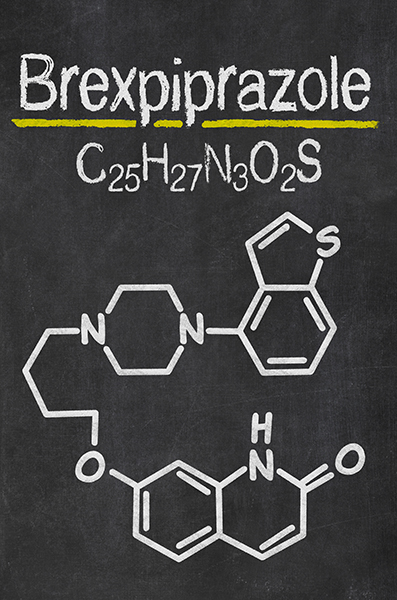
Brexpiprazole Warnings: Side Effects of Rexulti

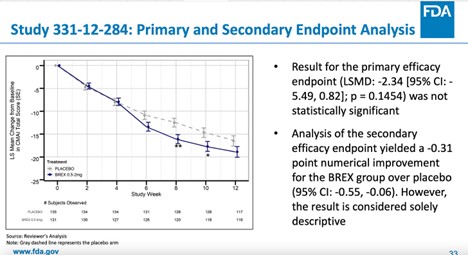
FDA rushes approval of dementia drug that quadruples risk of death

Regulatory - Page 7 of 12 - Drug Discovery World (DDW)

How the FDA approved an antipsychotic that failed to show a meaningful benefit but raised the risk of death

FDA Fast Track Designation for Ad-RTS-hIL-12 plus Veledimex for Recurrent Glioblastoma

Repurposed Drugs Offer a Fast(er) Track to Alzheimer's Treatment
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America
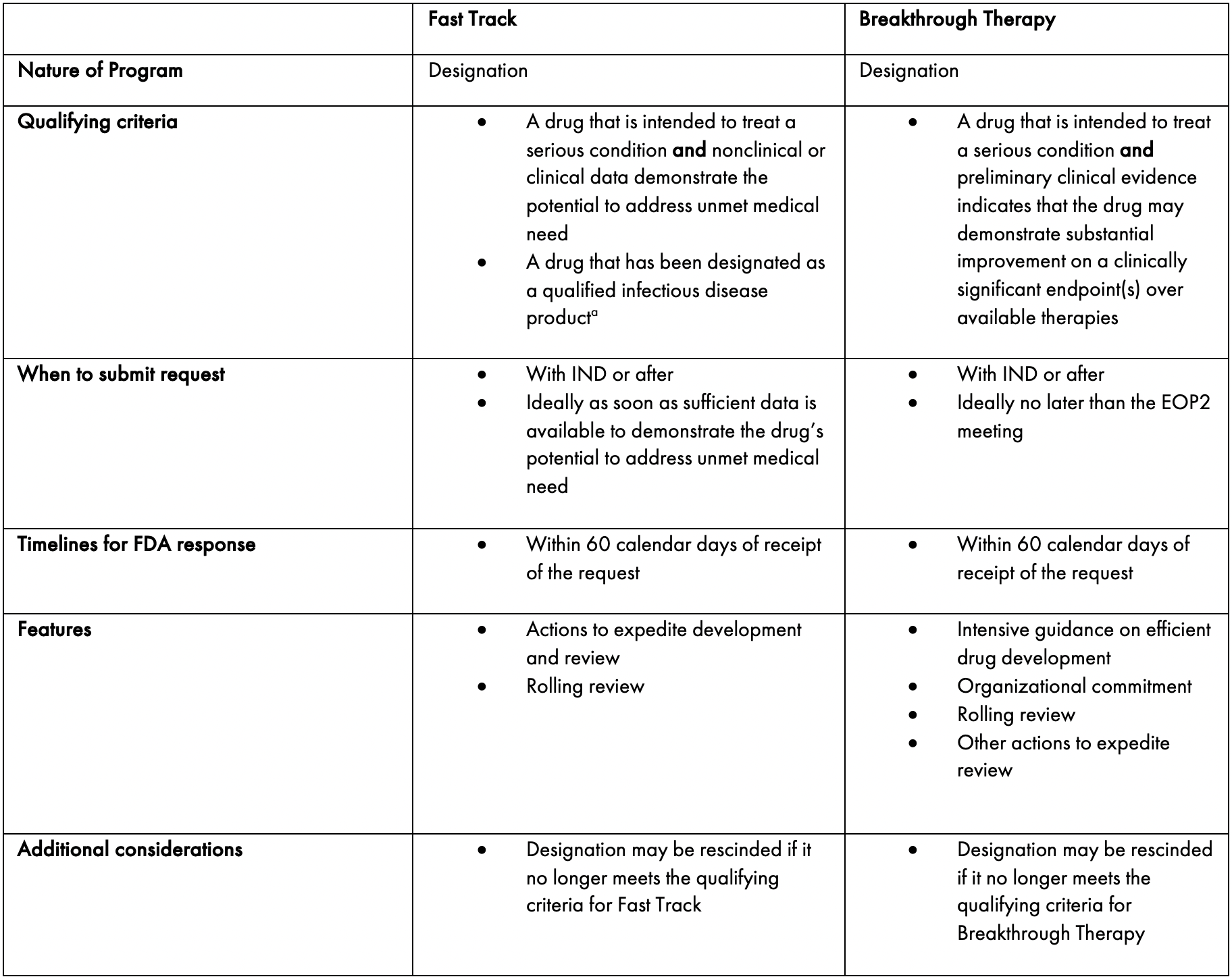
Fast Track Designation and Breakthrough Therapy Designation — Scendea

FDA Approves Rexulti For Agitation Associated With Dementia Due To Alzheimer's
FDA's Fast-Track for Rexulti Raises Concerns
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America
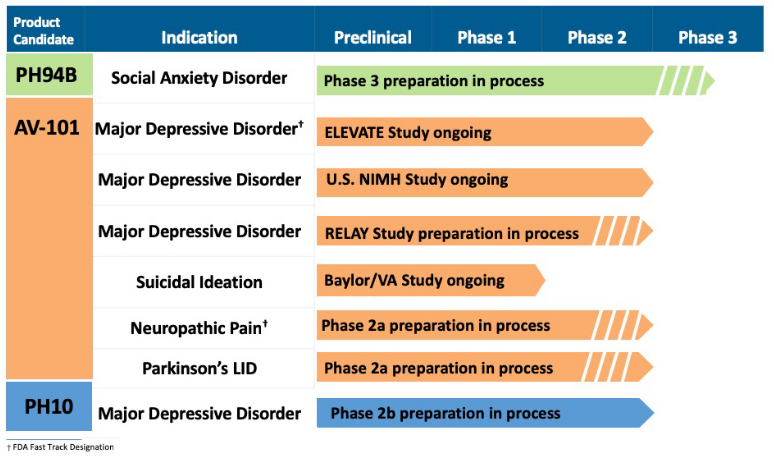
Vistgen And Relmada: Competitors In Depression Treatment (NASDAQ:RLMD)
Recomendado para você
-
 Otsuka America Pharmaceutical 59148003613 - McKesson Medical-Surgical11 novembro 2024
Otsuka America Pharmaceutical 59148003613 - McKesson Medical-Surgical11 novembro 2024 -
 Rexulti (Brexpiprazole) - PSYCH-MENTAL HEALTH HUB11 novembro 2024
Rexulti (Brexpiprazole) - PSYCH-MENTAL HEALTH HUB11 novembro 2024 -
 Rexulti 4 mg 28 tablets11 novembro 2024
Rexulti 4 mg 28 tablets11 novembro 2024 -
 Rexulti oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD11 novembro 2024
Rexulti oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD11 novembro 2024 -
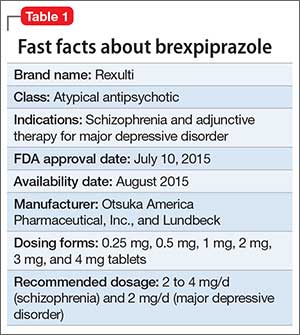 Brexpiprazole for schizophrenia and as adjunct for major depressive disorder11 novembro 2024
Brexpiprazole for schizophrenia and as adjunct for major depressive disorder11 novembro 2024 -
 Abilify vs. Rexulti: Similarities and differences11 novembro 2024
Abilify vs. Rexulti: Similarities and differences11 novembro 2024 -
 Rexulti copay card covers generics too : r/pharmacy11 novembro 2024
Rexulti copay card covers generics too : r/pharmacy11 novembro 2024 -
 Rexulti Review Effective for Schizophrenia and Depression? – Illuminate Labs11 novembro 2024
Rexulti Review Effective for Schizophrenia and Depression? – Illuminate Labs11 novembro 2024 -
 Compra Rexulti brexpiprazol 2 mg con 14 tabletas en Prixz11 novembro 2024
Compra Rexulti brexpiprazol 2 mg con 14 tabletas en Prixz11 novembro 2024 -
 REXULTI 2 MG Oral Tablet11 novembro 2024
REXULTI 2 MG Oral Tablet11 novembro 2024
você pode gostar
-
 UNCHARTED 4: A THIEF'S END - PS4 MÍDIA DIGITAL - LS Games11 novembro 2024
UNCHARTED 4: A THIEF'S END - PS4 MÍDIA DIGITAL - LS Games11 novembro 2024 -
 Wladimir Gramacho Deixem os macacos em paz: chamem só de varíola11 novembro 2024
Wladimir Gramacho Deixem os macacos em paz: chamem só de varíola11 novembro 2024 -
 ORDEN para ver BUNGOU STRAY DOGS - Orden Cronologico de Bungo Stray Dogs11 novembro 2024
ORDEN para ver BUNGOU STRAY DOGS - Orden Cronologico de Bungo Stray Dogs11 novembro 2024 -
 A Devil May Cry 5 The Game Awards 2018 Performance Has Been Announced - Siliconera11 novembro 2024
A Devil May Cry 5 The Game Awards 2018 Performance Has Been Announced - Siliconera11 novembro 2024 -
 Inside the Girls Club Room at the K-12 Chess Champs11 novembro 2024
Inside the Girls Club Room at the K-12 Chess Champs11 novembro 2024 -
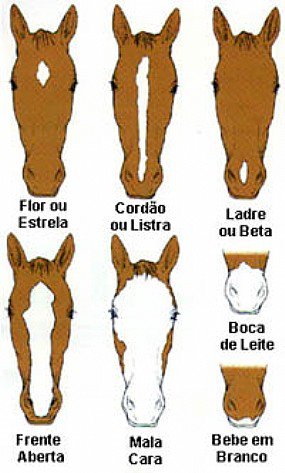 Sinais na face dos cavalos – Hipismo&Co11 novembro 2024
Sinais na face dos cavalos – Hipismo&Co11 novembro 2024 -
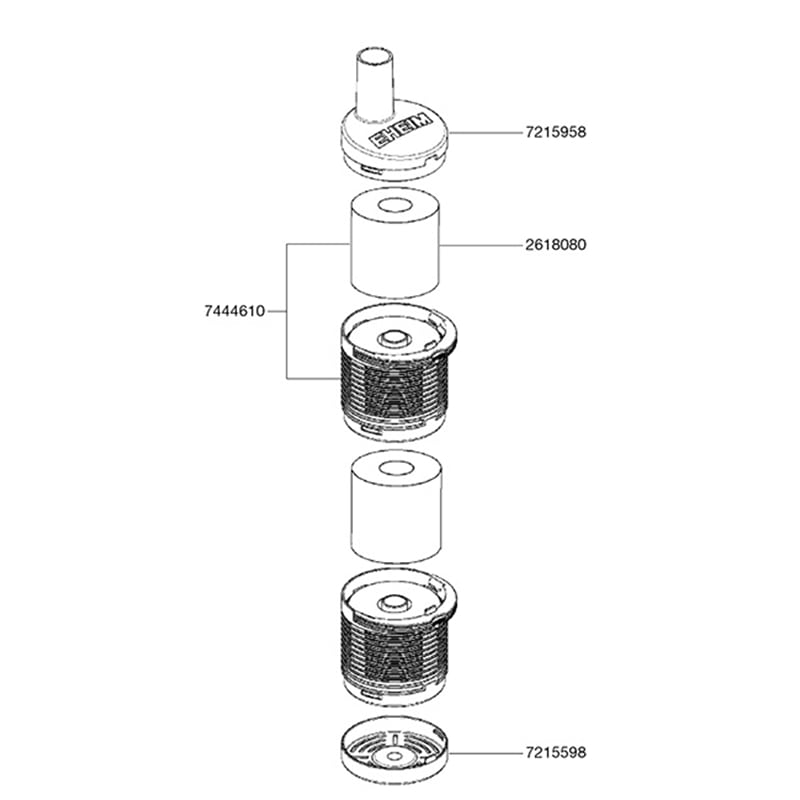 EHEIM Vorfilter11 novembro 2024
EHEIM Vorfilter11 novembro 2024 -
 One-Punch Man: Quando sai a 3ª temporada? Tudo o que já sabemos11 novembro 2024
One-Punch Man: Quando sai a 3ª temporada? Tudo o que já sabemos11 novembro 2024 -
melhores edits de anime luta|TikTok ရှာဖွေမှု11 novembro 2024
-
 Já podes ver Dragon Ball Z: Light of Hope, um novo filme feito por11 novembro 2024
Já podes ver Dragon Ball Z: Light of Hope, um novo filme feito por11 novembro 2024
