Otsuka and Lundbeck Announce U.S. Food and Drug Administration (FDA) Approval of Supplemental New Drug Application (sNDA) for REXULTI® (brexpiprazole) for the Treatment of Agitation Associated with Dementia Due to Alzheimer's Disease
Por um escritor misterioso
Last updated 23 abril 2025


FDA

Positive Phase III Alzheimer's results send Lundbeck and Otsuka

Lundbeck, Otsuka drug cut agitation in Alzheimer's patients

Allison Rosenthal on LinkedIn: So proud and humbled to announce a

Gate Neurosciences opens lab in NorthShore Evanston Hospital

Craig Chepke, MD, DFAPA on LinkedIn: Otsuka and Lundbeck Announce

Otsuka and Lundbeck gain FDA approval for supplemental New Drug

Julie Moore on LinkedIn: We work for the patients but also the

Mischa Agster on LinkedIn: Otsuka Announces New FDA Approval
FDA Approves First Drug to Treat Agitation Symptoms Associated
Recomendado para você
-
 REXULTI® (brexpiprazole) Patient Information Website23 abril 2025
REXULTI® (brexpiprazole) Patient Information Website23 abril 2025 -
 Rexulti FAQ - Prescription Drug Journal™23 abril 2025
Rexulti FAQ - Prescription Drug Journal™23 abril 2025 -
 Rexulti side effects and how to avoid them - NiceRx23 abril 2025
Rexulti side effects and how to avoid them - NiceRx23 abril 2025 -
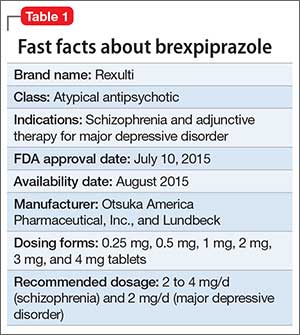 Brexpiprazole for schizophrenia and as adjunct for major depressive disorder23 abril 2025
Brexpiprazole for schizophrenia and as adjunct for major depressive disorder23 abril 2025 -
 Rexulti copay card covers generics too : r/pharmacy23 abril 2025
Rexulti copay card covers generics too : r/pharmacy23 abril 2025 -
 Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo23 abril 2025
Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo23 abril 2025 -
 New Drug Product: REXULTI - MPR23 abril 2025
New Drug Product: REXULTI - MPR23 abril 2025 -
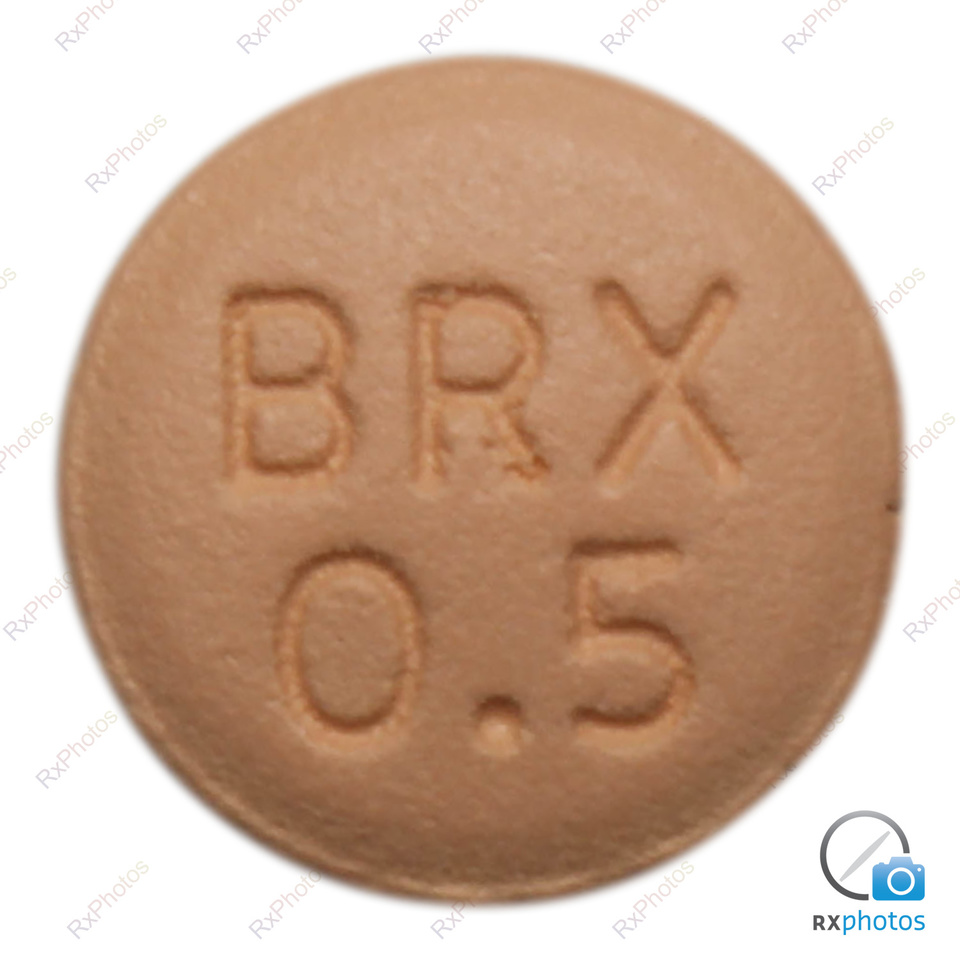
Rexulti tablet 0.5mg23 abril 2025
-
 Rexulti Alternatives We Offer Natural, Evidence-Based Options23 abril 2025
Rexulti Alternatives We Offer Natural, Evidence-Based Options23 abril 2025 -
 Buy Rx: Rexulti 1 mg Tablet Online23 abril 2025
Buy Rx: Rexulti 1 mg Tablet Online23 abril 2025
você pode gostar
-
Rexona - FOR EXCESSIVE SWEATING, Use New Rexona Clinical23 abril 2025
-
 Regina King on Oscar-Worthy Scenes in If Beale Street Could Talk23 abril 2025
Regina King on Oscar-Worthy Scenes in If Beale Street Could Talk23 abril 2025 -
 Street Fighter 6 release date and PC system requirements - The23 abril 2025
Street Fighter 6 release date and PC system requirements - The23 abril 2025 -
Pokemon GO Singapore My 1st Kangaskhan tapped from a passing cab in Sydney23 abril 2025
-
 O que é Otaku? Descubra porque ser um não é vergonhoso nem ofensivo23 abril 2025
O que é Otaku? Descubra porque ser um não é vergonhoso nem ofensivo23 abril 2025 -
 Dante Alighieri Inferno by walter giorgi23 abril 2025
Dante Alighieri Inferno by walter giorgi23 abril 2025 -
 Graphical Evolution of Sword Art Online Games (2012-2022)23 abril 2025
Graphical Evolution of Sword Art Online Games (2012-2022)23 abril 2025 -
 OptaJoao on X: 3 - Três jogadores sub-20 alcançaram os 10 gols no23 abril 2025
OptaJoao on X: 3 - Três jogadores sub-20 alcançaram os 10 gols no23 abril 2025 -
 Plastic Memories - Playstation Vita Game - Visual Novel - Limited Edition (5pb. Games, MAGES.)23 abril 2025
Plastic Memories - Playstation Vita Game - Visual Novel - Limited Edition (5pb. Games, MAGES.)23 abril 2025 -
 Deoxys Vs Mewtwo GX Pokemon Card23 abril 2025
Deoxys Vs Mewtwo GX Pokemon Card23 abril 2025