Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 23 abril 2025

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.
Early Drug Discovery and Development Guidelines: For Academic

Preclinical Working Group National Institutes of Health (NIH)

PDF) Molecular clinical safety intelligence: a system for bridging

PDF) Investigative safety strategies to improve success in drug
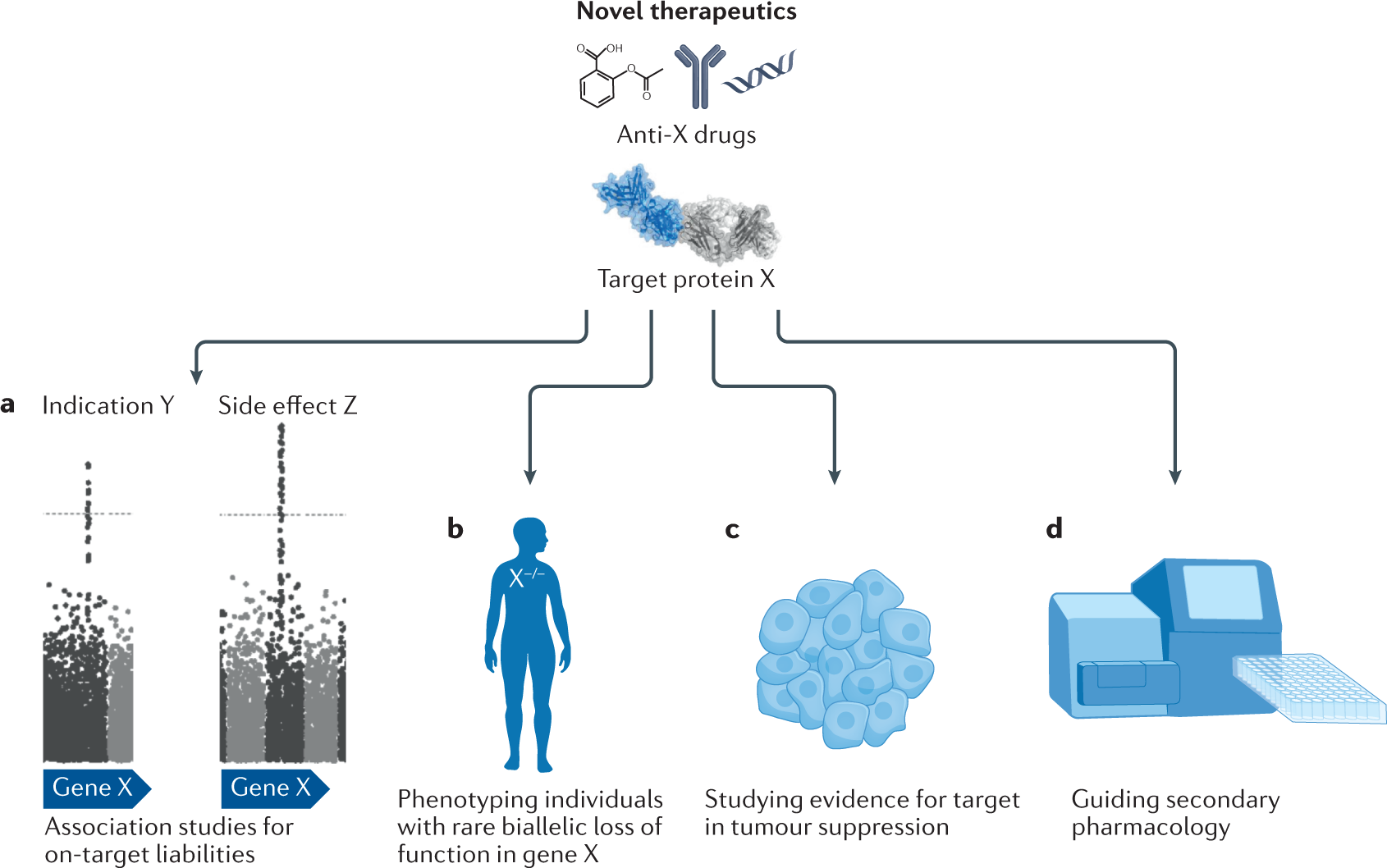
Using human genetics to improve safety assessment of therapeutics
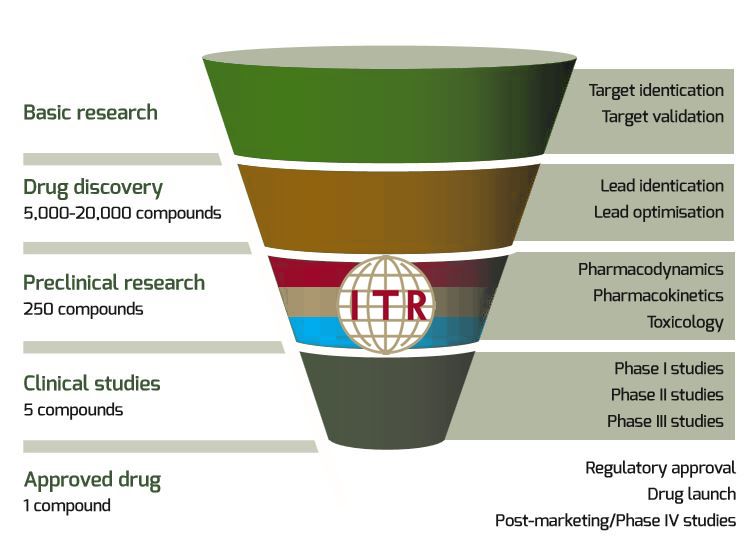
From Bench to Clinic - The Chronology of Preclinical Studies - ITR

Early Phase Drug Development Solutions

Artificial intelligence for drug discovery: Resources, methods
The workflow of drug discovery and development. Words in red font
Recomendado para você
-
 BRAIN TEST NÍVEL 411 EM PORTUGUÊS23 abril 2025
BRAIN TEST NÍVEL 411 EM PORTUGUÊS23 abril 2025 -
The University of Alabama's Brain-Drone Race Flies Us to a Mind23 abril 2025
-
 brain test nível 41123 abril 2025
brain test nível 41123 abril 2025 -
 braintest level 411|TikTok Search23 abril 2025
braintest level 411|TikTok Search23 abril 2025 -
 Brain Test Level 411 Jawaban - Games For Cats23 abril 2025
Brain Test Level 411 Jawaban - Games For Cats23 abril 2025 -
 Classification of autism spectrum disorder based on sample entropy23 abril 2025
Classification of autism spectrum disorder based on sample entropy23 abril 2025 -
 Got the pink shorthair! : r/CatsAndSoup23 abril 2025
Got the pink shorthair! : r/CatsAndSoup23 abril 2025 -
 ANAT 411 Anterior View of the Muscles of Head & Neck Diagram23 abril 2025
ANAT 411 Anterior View of the Muscles of Head & Neck Diagram23 abril 2025 -
Biosynthesis of Argolaphos Illuminates the Unusual Biochemical23 abril 2025
-
Anna Marie Vanderstelt-Frank on Instagram: ❤️Have you ever23 abril 2025
você pode gostar
-
 Roblox TROLLING In Rate My Avatar23 abril 2025
Roblox TROLLING In Rate My Avatar23 abril 2025 -
 Mapa De Natal Com Labirinto Jogo De Tabuleiro De Risco Para Crianças Boardgame No Estilo Cartoon Papai Noel, Veado, Pé-grande E E Ilustração do Vetor - Ilustração de educacional, cervos: 16433166323 abril 2025
Mapa De Natal Com Labirinto Jogo De Tabuleiro De Risco Para Crianças Boardgame No Estilo Cartoon Papai Noel, Veado, Pé-grande E E Ilustração do Vetor - Ilustração de educacional, cervos: 16433166323 abril 2025 -
 Play Arcade Power Drift (Japan) Online in your browser23 abril 2025
Play Arcade Power Drift (Japan) Online in your browser23 abril 2025 -
 Scp 939 Photographic Prints for Sale23 abril 2025
Scp 939 Photographic Prints for Sale23 abril 2025 -
Caminhoes Arqueados top's23 abril 2025
-
 Gate: Jieitai Kanochi Nite, Kaku Tatakaeri (Review) - Video Quest23 abril 2025
Gate: Jieitai Kanochi Nite, Kaku Tatakaeri (Review) - Video Quest23 abril 2025 -
 Jogo Lençol Infantil Gatinho 100% Algodão 3 Peças Santista23 abril 2025
Jogo Lençol Infantil Gatinho 100% Algodão 3 Peças Santista23 abril 2025 -
/cdn.vox-cdn.com/uploads/chorus_asset/file/16026625/jbareham_190410_ply0870_0094_mewtwo.jpg) Pokémon Go ultra rewards: Shiny Mewtwo, regionals, and Generation 5 in September - Polygon23 abril 2025
Pokémon Go ultra rewards: Shiny Mewtwo, regionals, and Generation 5 in September - Polygon23 abril 2025 -
 Hiiro Ryuugasaki (Shadowverse) - Pictures23 abril 2025
Hiiro Ryuugasaki (Shadowverse) - Pictures23 abril 2025 -
 Página 21, Vetores e ilustrações de Demon slayer para download gratuito23 abril 2025
Página 21, Vetores e ilustrações de Demon slayer para download gratuito23 abril 2025