What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 30 março 2025

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.

Overview of the IRB research review process.

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions
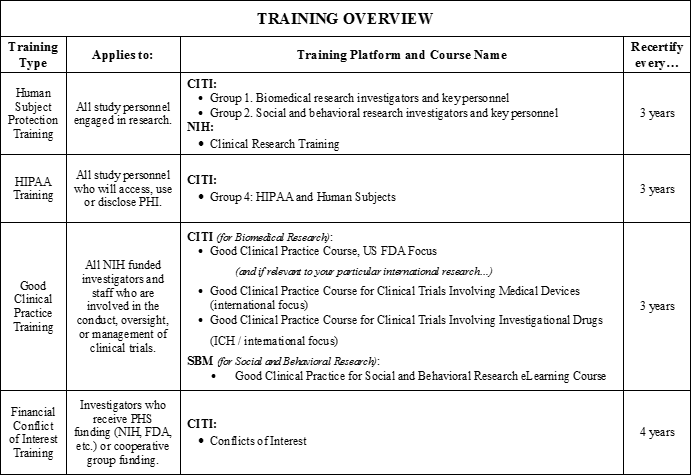
IRB 101, Office of Research Oversight/Regulatory Affairs
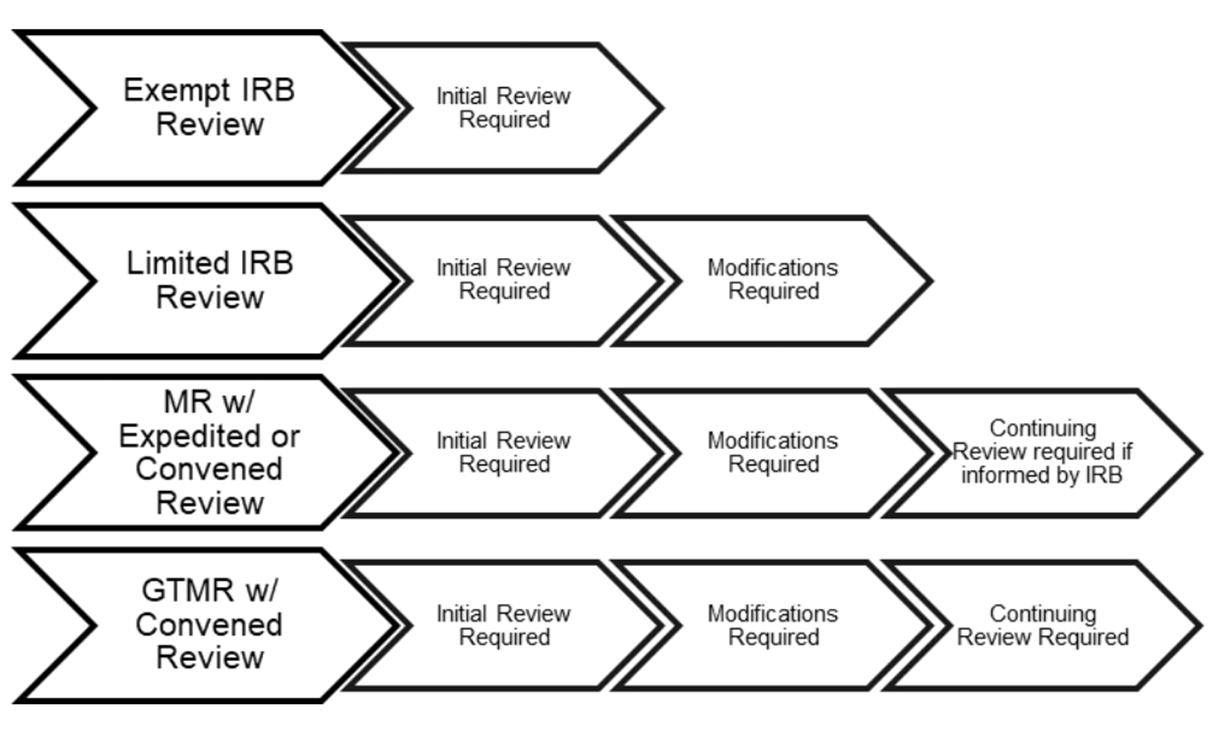
Penn IRB Levels of IRB Review - Penn IRB

PDF) The reporting Of IRB review in journal articles presenting HIV research conducted in the developing world

IRB Performance & Metrics

Do You Need IRB Review?

IRB Review Timeline - Office of Research Support and Compliance

IRB Review: How to
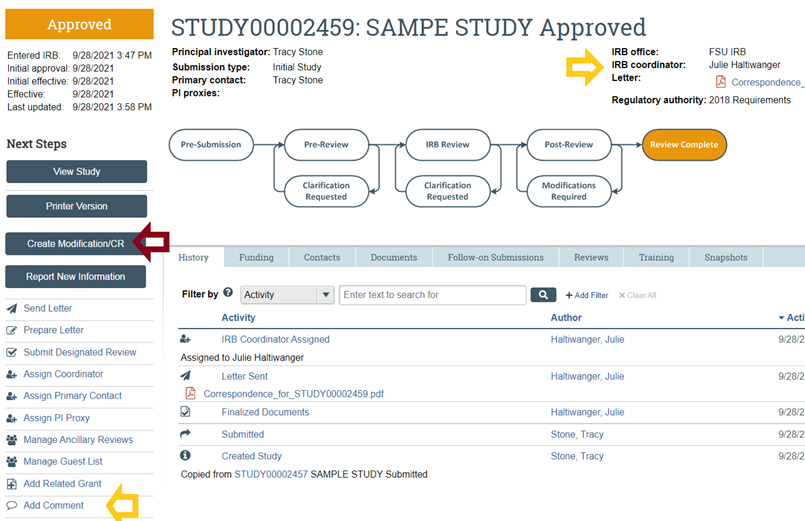
FAQs FSU Office of Research
New Common Rule, 2019, IRB Blog, Institutional Review Board
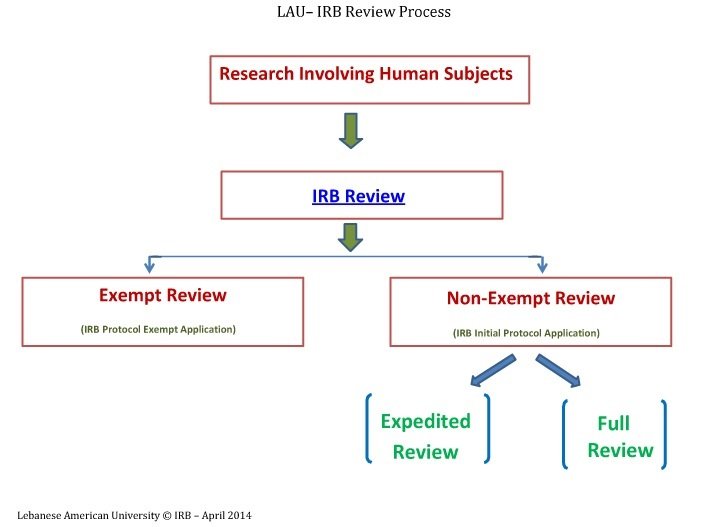
IRB Types of Review, Ethical Compliance, Graduate Studies and Research

Webinar: What You Should Know About IRB Review of Research
Recomendado para você
-
 صورة اخرى لنادي شبتين بعد حصوله على المركز الاول في دوري دير ابو مشعل 14\1\2011 - Shabtin - Ramallah - شبتين / شبطين (שיבתין) - Palestine Remembered30 março 2025
صورة اخرى لنادي شبتين بعد حصوله على المركز الاول في دوري دير ابو مشعل 14\1\2011 - Shabtin - Ramallah - شبتين / شبطين (שיבתין) - Palestine Remembered30 março 2025 -
 Instituto Rio Branco - IRB — Other em São Leopoldo30 março 2025
Instituto Rio Branco - IRB — Other em São Leopoldo30 março 2025 -
 irbsl.com.br at WI. IRBSL – Instituto Rio Branco30 março 2025
irbsl.com.br at WI. IRBSL – Instituto Rio Branco30 março 2025 -
 Irbsl (irbsl9201) - Profile30 março 2025
Irbsl (irbsl9201) - Profile30 março 2025 -
 Catalog - IRB Advisors, Inc.30 março 2025
Catalog - IRB Advisors, Inc.30 março 2025 -
 HISTÓRIA – IRBSL30 março 2025
HISTÓRIA – IRBSL30 março 2025 -
IR Labs Announces Strategic Partnership with Connection Silicon Valley30 março 2025
-
 IRBR3.SA -, Stock Price & Latest News30 março 2025
IRBR3.SA -, Stock Price & Latest News30 março 2025 -
 Yudia (@JoudiaBOUJDAINI) / X30 março 2025
Yudia (@JoudiaBOUJDAINI) / X30 março 2025 -
 Pew report: PA less restrictive on religion than Israel; Iran slightly worse30 março 2025
Pew report: PA less restrictive on religion than Israel; Iran slightly worse30 março 2025
você pode gostar
-
 Video 'Escape From Alcatraz' Mystery Revisited - ABC News30 março 2025
Video 'Escape From Alcatraz' Mystery Revisited - ABC News30 março 2025 -
 Great Eastern Entertainment Sonic The Hedgehog- Shadow Ball Plush 8 H,Multi-Colored,5221330 março 2025
Great Eastern Entertainment Sonic The Hedgehog- Shadow Ball Plush 8 H,Multi-Colored,5221330 março 2025 -
 Las tarjetas PlayStation llegaron a las tiendas argentinas - PressOver30 março 2025
Las tarjetas PlayStation llegaron a las tiendas argentinas - PressOver30 março 2025 -
 The Last of Us Part II - Joel Statue – Dark Horse Direct30 março 2025
The Last of Us Part II - Joel Statue – Dark Horse Direct30 março 2025 -
 Melhores jogos do Mario para curtir em família no Nintendo Switch - Save State30 março 2025
Melhores jogos do Mario para curtir em família no Nintendo Switch - Save State30 março 2025 -
 ebook Korn: Guia prático das traduções juramentadas - Korn30 março 2025
ebook Korn: Guia prático das traduções juramentadas - Korn30 março 2025 -
 Apple Watch Ultra Review: Pings for Days30 março 2025
Apple Watch Ultra Review: Pings for Days30 março 2025 -
 10 things you should know about 'Dead Space30 março 2025
10 things you should know about 'Dead Space30 março 2025 -
 Why Twitch Is Still the King of Live Game Streaming - The New York30 março 2025
Why Twitch Is Still the King of Live Game Streaming - The New York30 março 2025 -
Unblocked Games — New Tab30 março 2025
