What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 23 abril 2025

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

Understanding FDA's CSA Guidance in the Context of Current Regulations and GAMP® American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
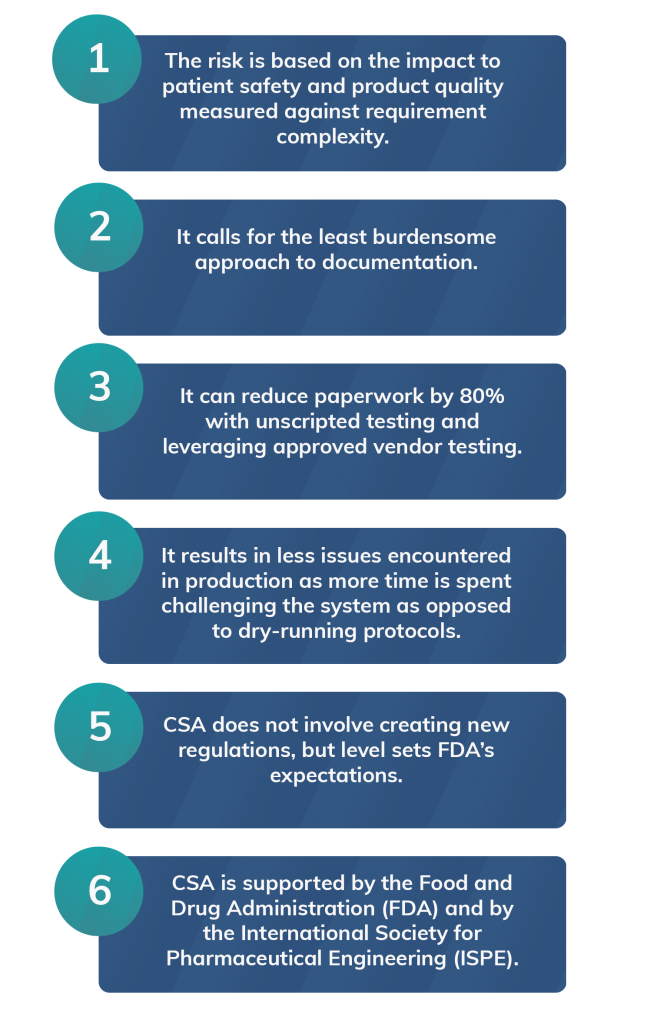
What is Computer Software Assurance (CSA) and why are the FDA transitioning from traditional Computer System Validation? - Kneat
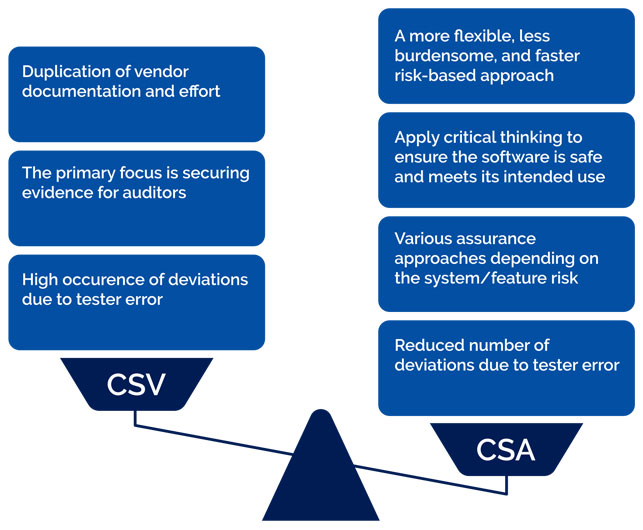
What is Computer Software Assurance (CSA), the FDA's new approach to CSV? - Vimachem

How To Complete Computer Systems Validation (FDA)

Software Validation: Here's How We Do It - Apprentice

Software Validation: Here's How We Do It - Apprentice
Recomendado para você
-
CSVP - MARANhHÃO23 abril 2025
-
Escola Deuzuita de Queiroz vai participar de Feira de Tecnologia23 abril 2025
-
 Merging Multiple CSV Files Using Pandas, by Prashant Gaur23 abril 2025
Merging Multiple CSV Files Using Pandas, by Prashant Gaur23 abril 2025 -
plugin-CsvImport/forms/Mapping.php at master · omeka/plugin23 abril 2025
-
 CSV File Format23 abril 2025
CSV File Format23 abril 2025 -
 Creating a csv file in other format than comma for master or23 abril 2025
Creating a csv file in other format than comma for master or23 abril 2025 -
 What is CSV?23 abril 2025
What is CSV?23 abril 2025 -
 CSVA-P (Power Cable) – Mirai Inter-Technologies23 abril 2025
CSVA-P (Power Cable) – Mirai Inter-Technologies23 abril 2025 -
CSVP (Colégio São Vicente de Paulo) - São Luís/MA - São Luís, MA23 abril 2025
-
 Highlighting the Latest Compute Security Capabilities in Prisma23 abril 2025
Highlighting the Latest Compute Security Capabilities in Prisma23 abril 2025
você pode gostar
-
 Epoch TV APK for Android Download23 abril 2025
Epoch TV APK for Android Download23 abril 2025 -
 What's the best place to get high quality drift mods? Either free or paid? : r/assettocorsa23 abril 2025
What's the best place to get high quality drift mods? Either free or paid? : r/assettocorsa23 abril 2025 -
Cut the Rope APK (Android Game) - Free Download23 abril 2025
-
/i.s3.glbimg.com/v1/AUTH_bc8228b6673f488aa253bbcb03c80ec5/internal_photos/bs/2020/5/H/JtVVoWQJANzLGnFWhb6Q/bloco-b-1.jpg) Campeonato do Alok: formato, equipes, datas e premiações do23 abril 2025
Campeonato do Alok: formato, equipes, datas e premiações do23 abril 2025 -
 Is Lego Star Wars The Skywalker Saga Multiplayer?23 abril 2025
Is Lego Star Wars The Skywalker Saga Multiplayer?23 abril 2025 -
 Pokémon Diamante & Pérola, Dublapédia23 abril 2025
Pokémon Diamante & Pérola, Dublapédia23 abril 2025 -
 Power Rangers Fúria Cósmica – Baixar Series MP423 abril 2025
Power Rangers Fúria Cósmica – Baixar Series MP423 abril 2025 -
Matching outfit idea under 100 Robux!! 🐶🐱 User: alxyvi (outfits save, 80 robux girl outfit23 abril 2025
-
 Unknown - Puff Daddy with Sunglasses Vintage Original Polaroid For Sale at 1stDibs p diddy sunglasses the four, puff daddy sunglasses, p diddy sunglasses on the four23 abril 2025
Unknown - Puff Daddy with Sunglasses Vintage Original Polaroid For Sale at 1stDibs p diddy sunglasses the four, puff daddy sunglasses, p diddy sunglasses on the four23 abril 2025 -
 Vlad Garfunkel MBTI Personality Type: INTJ or INTP?23 abril 2025
Vlad Garfunkel MBTI Personality Type: INTJ or INTP?23 abril 2025


