Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Por um escritor misterioso
Last updated 13 março 2025
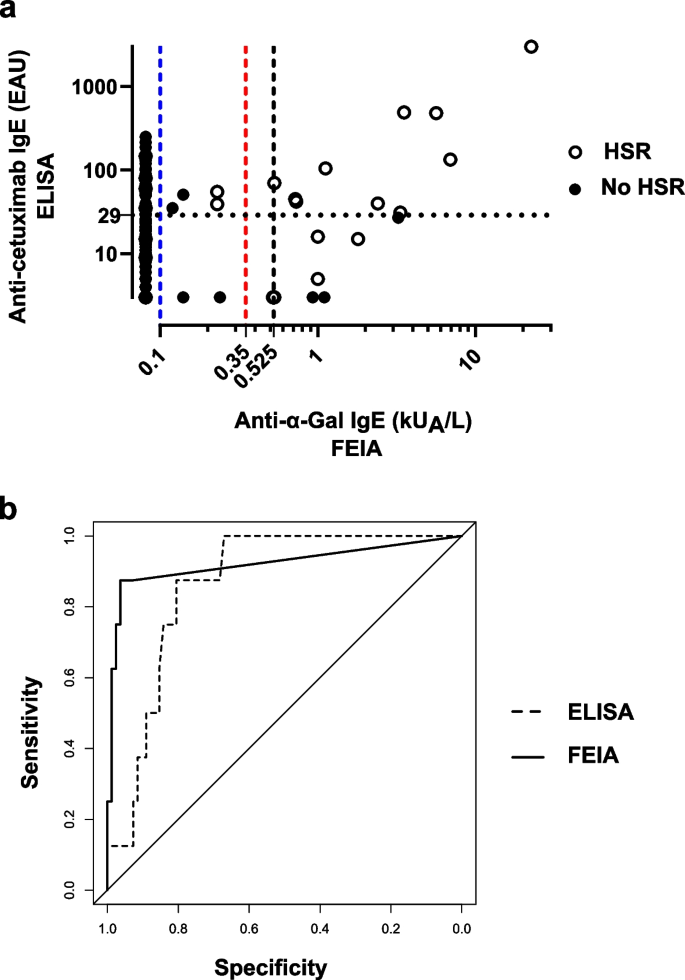
Background The link between immediate hypersensitivity reactions (HSR) following the first cetuximab infusion and the IgE sensitization against anti-galactose-α-1,3-galactose (α-Gal) is now well-established. An automated Fluoroenzyme-Immunoassay (FEIA) is available and may facilitate the screening of patients with anti-α-Gal IgE before treatment. Methods This study aimed to evaluate its performances as compared to a previously validated anti-cetuximab IgE ELISA, using 185 samples from two previously studied cohorts. Results Despite 21.1% of discrepancies between the two techniques, FEIA discriminated better positive patients and similarly negative ones with a ≥ 0.525 kUA/L threshold. Sensitivity was 87.5% for both tests, specificity was better for FEIA (96.3% vs ELISA: 82.1%). FEIA had a higher positive likelihood ratio (23.9 vs ELISA: 4.89) and a similar negative likelihood ratio (0.13 vs ELISA: 0.15). In our population, the risk of severe HSR following a positive test was higher with FEIA (56.7% vs ELISA: 19.6%) and similar following a negative test (0.7% vs ELISA: 0.8%). Conclusion Although the predictive value of the IgE screening before cetuximab infusion remains discussed, this automated commercial test can identify high-risk patients and is suitable for routine use in laboratories. It could help avoiding cetuximab-induced HSR by a systematic anti-α-Gal IgE screening before treatment.
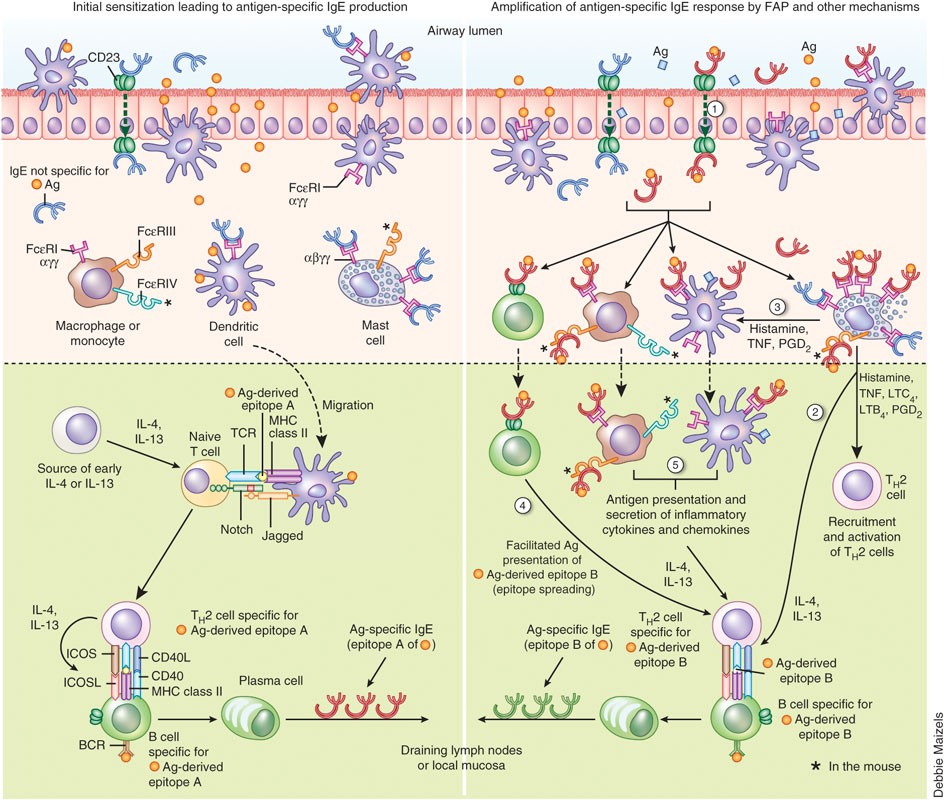
IgE and mast cells in allergic disease

Prevalence of anti-cetuximab IgE. IgE levels were measured in serum

Alpha-Gal-containing biologics and anaphylaxis - ScienceDirect

BMC Cancer 1/2023

Full article: Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS)
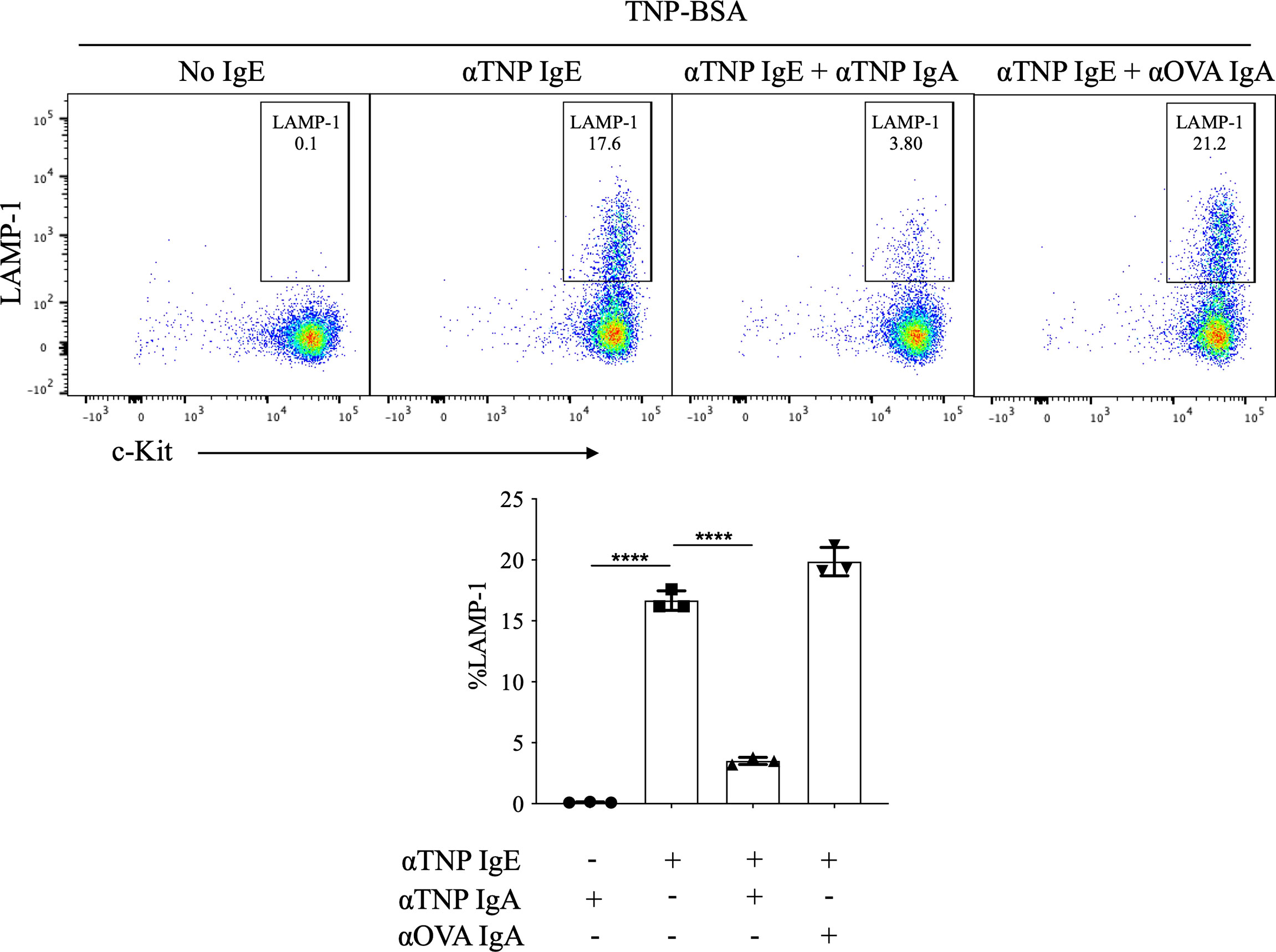
Frontiers Allergen-Specific IgA Antibodies Block IgE-Mediated Activation of Mast Cells and Basophils
Solved] Please see the question below. A physician ordered a type and

Description of the study population. HSR: Hypersensitivity reaction.

Anti-IgE as a mast cell–stabilizing therapeutic agent - ScienceDirect

Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer

Risk of bias and applicability concerns summary.

The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization - ScienceDirect
Recomendado para você
-
 De Frozen: veja as 14 Elsas mais bizarras que estão espalhadas pelo mundo! - Purebreak13 março 2025
De Frozen: veja as 14 Elsas mais bizarras que estão espalhadas pelo mundo! - Purebreak13 março 2025 -
 Kkkkkkk ( elsa feia estou kkkk) SUERA13 março 2025
Kkkkkkk ( elsa feia estou kkkk) SUERA13 março 2025 -
 Frozen Quizur13 março 2025
Frozen Quizur13 março 2025 -
 La reacción viral de una niña tras recibir una torta fallida de la película Frozen13 março 2025
La reacción viral de una niña tras recibir una torta fallida de la película Frozen13 março 2025 -
 Receita de Bolo Confeitado, enviada por tereza cristina da silva - TudoGostoso13 março 2025
Receita de Bolo Confeitado, enviada por tereza cristina da silva - TudoGostoso13 março 2025 -
 Hedy Hopper - casper's scare school Photo (37693738) - Fanpop13 março 2025
Hedy Hopper - casper's scare school Photo (37693738) - Fanpop13 março 2025 -
 Página 4 Fotos Hediondo, 300+ fotos de arquivo grátis de alta qualidade13 março 2025
Página 4 Fotos Hediondo, 300+ fotos de arquivo grátis de alta qualidade13 março 2025 -
Quem é a princesa mais feia do mundo? - Quora13 março 2025
-
 The Crowd - 1 by TrashChameleon on DeviantArt13 março 2025
The Crowd - 1 by TrashChameleon on DeviantArt13 março 2025 -
Boneca Feia em Promoção na Shopee Brasil 202313 março 2025
você pode gostar
-
 Their daughter nearly drowned. Now they're going into debt paying for her care13 março 2025
Their daughter nearly drowned. Now they're going into debt paying for her care13 março 2025 -
/i.s3.glbimg.com/v1/AUTH_bc8228b6673f488aa253bbcb03c80ec5/internal_photos/bs/2019/G/N/V6Rt90TVqs9KabrLwXLg/gettyimages-867506056.jpg) Fifa anuncia que Mundial sub-17, no Brasil, será disputado de 2613 março 2025
Fifa anuncia que Mundial sub-17, no Brasil, será disputado de 2613 março 2025 -
 Actor Lance Reddick from Quantum Break died today 💔 : r/controlgame13 março 2025
Actor Lance Reddick from Quantum Break died today 💔 : r/controlgame13 março 2025 -
 Grand Opening English Icon Text Design Vector Halftone, Grand Opening English, Grand Opening Banner, Grand Opening Text PNG and Vector with Transparent Backgrou…13 março 2025
Grand Opening English Icon Text Design Vector Halftone, Grand Opening English, Grand Opening Banner, Grand Opening Text PNG and Vector with Transparent Backgrou…13 março 2025 -
 Granblue Fantasy: Relink - PlayStation Showcase Trailer13 março 2025
Granblue Fantasy: Relink - PlayStation Showcase Trailer13 março 2025 -
Edu Parcão Voleibol - 1o Torneio Internacional de Voleibol Master - Sogipa 50+ Masculino Campeão!! @eduparcaovoleibol #volleybal #volleyball #voleimasculino #voleidequadra13 março 2025
-
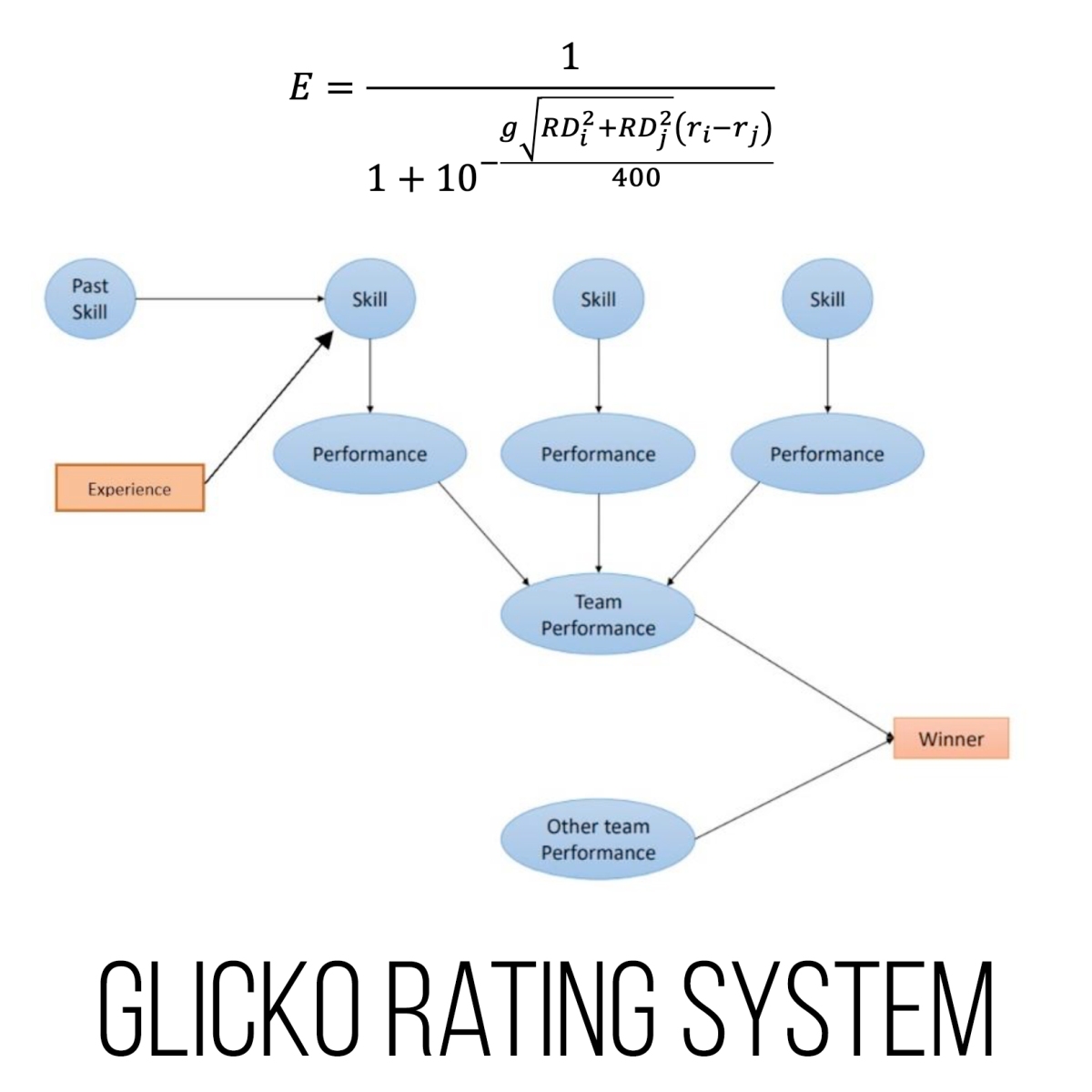 Elo and Glicko Standardised Rating Systems – TOM ROCKS MATHS13 março 2025
Elo and Glicko Standardised Rating Systems – TOM ROCKS MATHS13 março 2025 -
:max_bytes(150000):strip_icc()/How-To-Train-Like-Scarlett-Johansson-in-Black-Widow-GettyImages-1205142102-2000-0b911fbae01e4ad2aefe5615eaae37cd.jpg) Scarlett Johansson's Trainer Reveals How to Follow Her 'Black Widow' Workout Routine13 março 2025
Scarlett Johansson's Trainer Reveals How to Follow Her 'Black Widow' Workout Routine13 março 2025 -
 Double Dragon Gaiden: Rise of the Dragons - Review - NookGaming13 março 2025
Double Dragon Gaiden: Rise of the Dragons - Review - NookGaming13 março 2025 -
 Samsung S23 Ultra review: Our verdict on the Android flagship13 março 2025
Samsung S23 Ultra review: Our verdict on the Android flagship13 março 2025

